Acidification
Ocean Acidification
General
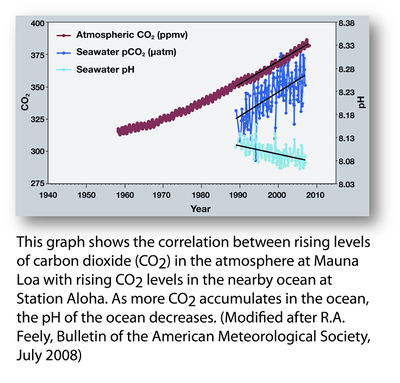
Ocean Acidification is defined as the process by which the acidity of ocean water increases, as pH lowers, due to an increase in the burning of fossil fuels by human industrialization. This causes an increase in carbon dioxide in the atmosphere, which is then absorbed by the ocean, leading to an imbalance in the chemistry of ocean water, which results in the pH lowering. This process has severe implications for marine ecosystems.
Mechanism
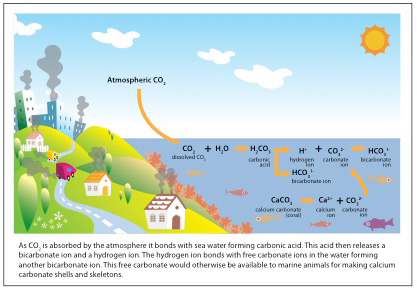
Ocean Acidification results as the equilibrium of oceanic chemistry is disrupted when increasing atmospheric carbon dioxide is absorbed by the ocean and reduces the pH of the water. This occurs through a chemical intensive process. Initially, carbon dioxide reacts with water to form carbonic acid. The carbonic acid further reacts to create bicarbonate and a hydrogen ion, which increases the acidity of water. The extra hydrogen ions then bond with the carbonate ions that are in the water, reducing the amount of carbonate available in the ocean.
The process is driven by the burning of fossil fuels by humans that results in an increase in carbon dioxide in the atmosphere. Most human activity: driving, industry, food production, and more, are all carbon emitting processes that add to the amount of carbon dioxide in the atmosphere. [1] About one quarter of the carbon dioxide that we release through these processes is absorbed by the ocean. This means that the actions we take on land do not just affect our terrestrial environments, but extend through the atmosphere to the ocean waters. [2]
Effects on Coral Reefs
Ocean acidification can have a lot of effects on the ocean ecosystems. Coral Bleaching is one potential effect that results when corals lose their zooxanthellae due to altering environmental factors. Zooxenthellae are the symbiotic algae that reside in coral colonies and provide them with the ability to perform photosynthesis and gain energy.

Without these zooxanthellae, the coral colonies cannot continue to grow and form reefs. Therefore, this can have very detrimental effects not only for the coral colonies themselves, but also for the diverse ecosystems that rely on the coral infrastructure. Usually bleaching is due to temperature variances in water around the corals; however, other factors, such as increased carbon dioxide, can also contribute. In one study, high carbon dioxide doses led to 40%-50% bleaching of coral colonies in a sample population. In the same study, carbon dioxide and acidity were shown to sometimes be more effective at bleaching than temperature; however, when temperature and carbon dioxide increases are combined, massive bleaching events can occur.
Other effects on corals can include a reduction of photosynthesis activity even when the corals are not bleached. In other words, even small changes in water chemistry, can disturb the coral, whose response is to perform less photosynthesis. Again, this means that the corals can not obtain any energy and therefore are stranded without any means for growing. [3]
Another major effect of ocean acidification is a reduction in calcification. This can lead to decreased linear extension rates and reduction of skeletal density, so that either the corals grow slower than usual or they begin to form less dense calcium carbonate skeletons. A less dense skeleton can lead to an increase in grazing damage by organisms and turbulence as the skeleton is much weaker. Finally, the acidification can lead corals to expend a greater amount of energy on calcification because there are less carbonate ions in the water for the corals to use as part of their skeletons. These factors add up to make it especially hard for the corals because they not only experience a loss of energy intake, but also require more energy to make their skeletons. [1]
A 2016 study found "that ocean acidification (PCO2 ~900 μatm, pH ~7.7) not only causes reduced overall mineral deposition but also a deformed and porous skeletal structure in newly settled coral recruits." [4]
Where it's happening
- Location based studies on Ocean Acidification
- 1) One area where OA has become an increasing problem is in the Caribbean. Ocean acidity is making coral vulnerable to erosion and natural phenomenon that are detrimental to reefs.[5] Located between North and South America, it is an area that is more affected by man-made acidification. Runoff and increased carbon emissions are an unfortunate externality of industrialized countries. OA levels in the Caribbean have already seen a 30-fold increase according to research by Nature Climate Change. [6]
- 2) The Coral Triangle, north of Australia, has seen an evident rise in the disintegration of skeletons of hermatypic corals and shellfish.[7] These coral reefs have been tremendously impacted by increased sea temperatures and coral bleaching. Rising sea levels are also becoming a problem, flooding the mangroves and endangering the juvenile fish populations that live within them.[8]
- 3) A collection of carbonate chemistry data on the Florida Reef Tract shows a decrease in the aragonite saturation values that reduce calcification rates of organisms.[9]While corals response to changing CO2 levels varies by organism, the overall trend is that it is negatively affecting their growth rates. The oceans absorb between 30-50% of atmospheric CO2, making this reef so close to the coast of South Florida highly susceptible to the problems caused by growing carbon emissions.
- 4) Sampling of Porites coral colonies in the Great Barrier Reef reveals a decrease in growth of 13% across the reef. It is easier to track the changing rates of calcification in Porites because they grow in patterns similar to most trees, with ring-like layers. Scientists can observe the thickness of the layers and see that in the past decades they have been growing thinner and thinner.[10]
- 5) Tyyrhenian Sea now has a declining variety or absence of organisms previously abundant in the area. Surrounding the small island of Castello Aragonese there is evidence of seawater corrosion from rising CO2 levels. CO2 bubbles rise from the seafloor, from volcanic activity below the surface. This bubbling activity has been occurring for centuries but is only intensified by the gradual rise in CO2 levels from the burning of fossil fuels that is happening globally.[11]
Looking Forward
Ocean acidification is not a process that will naturally end. Because we continue to emit carbon dioxide this problem will continue. Therefore, it is necessary for us to understand how we can slow down and prevent these effects on reef ecosystems. Preventing future ocean acidification and reducing the harmful effects that it has on coral reefs starts by realizing that a large percent of CO2 emissions are emitted from urban areas. Reducing the population's carbon footprint is something that each individual can work to improve. Collecting seawater CO2 level data, monitoring urban and agricultural runoff, and starting citywide programs to reduce greenhouse gas emissions are a few of the steps being taken to counteract this growing problem. In addition, nationwide and even global programs for reducing carbon dioxide emissions are important in preventing further acidification of our ocean ecosystems. [12]
A new study [13] suggests that "ocean acidification may cause dramatic changes to phytoplankton". The paper, "Impact of ocean acidification on the structure of future phytoplankton communities", can be found in Nature Climate Change (2015) doi:10.1038/nclimate2722 http://www.nature.com/nclimate/journal/vaop/ncurrent/full/nclimate2722.html .
References
- ↑ 1.0 1.1 Hoegh-Guldberg, Ove, et al. "Coral reefs under rapid climate change and ocean acidification." science 318.5857 (2007): 1737-1742. http://www.sciencemag.org/content/318/5857/1737.short
- ↑ NOAA "Ocean Acidification." Ocean Acidification. NOAA PMEL Carbon Program, n.d. Web. 26 Feb. 2014. http://pmel.noaa.gov/co2/story/Ocean+Acidification
- ↑ Anthony, Kenneth RN, et al. "Ocean acidification causes bleaching and productivity loss in coral reef builders." Proceedings of the National Academy of Sciences 105.45 (2008): 17442-17446. http://www.pnas.org/content/105/45/17442.short
- ↑ T. Foster, J. L. Falter, M. T. McCulloch, P. L. Clode, Ocean acidification causes structural deformities in juvenile coral skeletons. Sci. Adv. 2, e1501130 (2016). http://advances.sciencemag.org/content/2/2/e1501130
- ↑ "We Need Your Help!" NOAA. N.p., 21 Nov. 2008. Web. 26 Feb. 2014.
- ↑ "Rising Ocean Acidity Worst for Caribbean and Pacific - SciDev.Net." SciDevNet. N.p., 7 Feb. 2012. Web.
- ↑ "The Coral Triangle- Oasis of Life." Coral Reef Science Made Accessible. N.p., n.d. Web. 26 Feb. 2014.
- ↑ "Climate Change, Reefs and the Coral Triangle." WWF Global. N.p., n.d. Web.
- ↑ "Ocean Acidification Refugia of the Florida Reef Tract." PLOS ONE:. N.p., 27 July 2012. Web. 26 Feb. 2014.
- ↑ "Ocean Acidification Hits Great Barrier Reef." Scientific American Global RSS. N.p., 1 Jan. 2009. Web. 26 Feb. 2014.
- ↑ "Ocean Acidification." National Geographic. N.p., Apr. 2011. Web. 26 Feb. 2014.
- ↑ "What Can We Do about Ocean Acidification?" NOAA Ocean Acidification Program. N.p., n.d. Web. 23 Feb. 2014.
- ↑ http://phys.org/news/2015-07-ocean-acidification-phytoplankton.html